Pharmaceuticals have an extremely important and indispensable position and role in social life. Presently, pharmaceuticals are not just a common commodity but have become an essential need of life. Pharmaceuticals play a role in treating various diseases, preventing and suppressing diseases, and enhancing the health of users. Certificate of Eligibility for pharmacy business helps the Government and Management Agencies manage product quality and evaluate the production process of different types of pharmaceuticals. In the article below, Viet An Law Firm will consolidate legal knowledge about Certificate of Eligibility for pharmacy business in Vietnam.
Table of contents
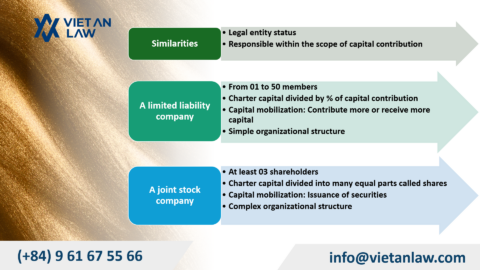
Pharmaceutical business is a commercial activity related to the production, purchase, import, export, distribution, and advertising of pharmaceutical products. Pharmaceuticals are products used for health care and disease treatment, including drugs, vitamins, minerals, herbs, and other related products.
The person in charge of pharmacy expertise and the persons holding the positions must have pharmacy practice certificates suitable for pharmacy business establishments.
The assessment of infrastructure, equipment, and personnel shall be carried out every 03 years or on an ad hoc basis under regulations of the Minister of Health or international treaties to which Vietnam is a signatory.
| Step 1 | The establishment applying for a Certificate of Eligibility for pharmaceutical business shall send documents to the Department of Health where the establishment’s business location is located. |
| Step 2 | After receiving the application, the Department of Health returns to the applicant for the Certificate of Eligibility for pharmaceutical business the application receipt according to Form No. 01 Appendix I of Decree No. 54/2017/ND-CP. In case there is no request to amend or supplement applications, the Department of Health:
· Issue the Certificate of Eligibility for pharmaceutical business within 20 days from the date recorded on the application receipt form in cases where the facilities, techniques, and personnel have been inspected and evaluated to meet the requirements of appropriate good practice to the scope of business, not having to organize an actual assessment at the facility applying for a Certificate of Eligibility for pharmaceutical business. · Organize an on-site assessment at the facility requesting the issuance of a Certificate of Eligibility for pharmaceutical business within 20 days from the date recorded on the Application Receipt Form. In case there is a request to amend or supplement the dossier, within 10 working days from the date recorded on the dossier receipt, the Department of Health will send a document to the facility requesting it, which must specifically state documents and content that need to be amended and supplemented. |
| Step 3 | After receiving the amended and supplemented dossier, the Department of Health shall return to the application receipt form of the amended and supplemented dossier according to Form 01 in Appendix I issued with Decree 54/2017/ND-CP.
· In case the amended or supplemented dossier does not meet the requirements, the Department of Health will notify the facility in writing; · In case, there is no request to amend or supplement the amended or supplemented dossier, the Department of Health shall comply with the law. |
| Step 4 | After assessing the actual facility, the Department of Health is responsible for:
· Issue a Certificate of Eligibility for pharmaceutical business within 10 working days from the date of completion of the actual assessment in cases where there are no requests, remedies or repairs; · Issue a written notice of the contents that need to be remedied or repaired within 05 working days from the date of completion of the actual assessment in case of requests, remedies or repairs. |
| Step 5 | Within 20 days from the date of receipt of the written notice and documents proving the completion of remedies and repairs of the requesting facility, the Department of Health shall issue a Certificate of Eligibility for pharmaceutical business or respond to the reason for not granting. |
| Step 6 | Within 05 working days from the date of issuance of the Certificate of Eligibility for pharmaceutical business, the Department of Health shall publish and update the following information on the electronic portal:
· Name and address of the facility issued with the Certificate of Eligibility for pharmaceutical business; · Full name of person responsible for pharmacy expertise, Pharmacy Practice Certificate number; · Number of Certificates of Eligibility for pharmaceutical business |
According to Article 40 of Pharmacy Law in 2016, cases in which the Certificate of Eligibility for pharmacy business is issued as follows:
Clients who have questions related to the Certificate of Eligibility for pharmacy business in Vietnam, please contact Viet An Law Firm for the best advice!