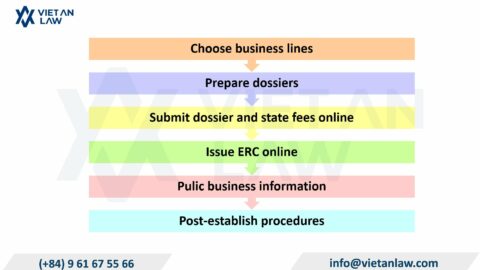
On September 25, 2023, the Minister of Health issued Circular No. 17/2023/TT-BYT amending, supplementing, and abolishing a number of legal documents on food safety that are no longer consistent with the practical conditions. Viet An Law Firm will present the amendment of Circular 17/2023/TT-BYT updates documents in the field of food safety in the article below.
According to the Ministry of Health, the issuance of Circular No. 17/2023/TT-BYT is very timely. The Circular was created with the purpose of amending and abolishing documents and regulations that are no longer suitable for practical conditions, ensuring the unity and synchronization of the legal system, and helping to save time and effort. capacity and promptly respond to management requirements. In addition, the Circular also ensures simplicity, transparency, ease of understanding, and implementation, creating favorable conditions for organizations and individuals operating in the food sector, and contributing to promoting administrative procedure reform.
Regarding transitional provisions, Circular 17/2023/TT-BYT clearly states that food processing aids and foods using food processing aids have been granted a certificate of declaration of conformity or certificate of conformity. If you receive a declaration of compliance with food safety regulations before the effective date of this Circular, you may continue to use it until the expiration date stated in the receipt or confirmation or the expiration date of the product.
Food processing aids, foods using food processing aids that have self-declared or registered a product declaration before the effective date of this Circular if not in accordance with the provisions of this Circular will continue to be used until the end of the product’s shelf life.
Regarding the terms of reference, in case the legal documents referenced for application in this Circular are amended, supplemented, or replaced, the amended, supplemented, or replaced documents shall apply.
Notably, this Circular supplements the list of flavorings used in food, including flavors recorded in the list that have been evaluated by JECFA or recognized as safe by the Food Manufacturers Association. United States flavors and extracts or flavorings for use in European Union foods issued by the Parliament and the Council of the European Union. In addition, 18 food additives are added with limited maximum usage limits, details in Appendix 2B and a new List of processing aids allowed to be used in food.
Abolish all documents of Circular 14/2011/TT-BYT dated April 1, 2011, guiding sampling for inspection and testing of food quality, hygiene, and safety.
Regarding the partial annulment of documents, specifically as follows:
Abolish some contents of the Regulation on maximum limits of biological and chemical contamination in food issued together with Decision 46/2007/QD-BYT. Here, abolish:
Specifically, according to Point b, Clause 2, Article 7, Circular 17/2023/TT-BYT updating documents in the field of food safety, abolishing Points b, c, d, and dd, Clause 10, Article 1 in this Circular.
Here, Circular 17/2023/TT-BYT stipulates the abolition of Point dd, Clause 1, Article 6 in Circular 48/2015/TT-BYT regulating food safety inspection activities in production and business. Food is under the management of the Ministry of Health. Accordingly, the inspection content will no longer include issues related to periodic product testing for food production and business establishments.
In Clause 1, Article 3, Circular 17/2023/TT-BYT updating documents in the field of food safety stipulates the application of good manufacturing practices (GMP) and certification equivalent to the Facility Certificate. meet food safety conditions to meet GMP requirements for imported health protection foods. Specifically:
Imported health protection foods must be produced at a facility that has been approved or recognized by a competent authority of the producing country or an organization designated by a competent authority or an agency or organization of another country. The competent authority of the manufacturing country shall grant one of the following certificates of product type suitable to the type of imported health protection food product: (i) Certificate of good manufacturing practice (GMP) for facilities manufacturing health protection food products; (ii) Certificate or assessment meeting good manufacturing practices (GMP) for drug or food manufacturing facilities;
For countries or territories that do not issue the certificates specified in Points a and b, Clause 1 of this Article, organizations and individuals must submit a certificate that contains one of the following contents: (i ) conforms to Hazard Analysis and Critical Control Point (HACCP – Hazard Analysis and Critical Control Point) standards; (ii) conform to ISO 22000 standard (International Organization for Standardization 22000); (iii) conform to international food standards (IFS – International Food Standard); (iv) in accordance with global food safety standards of the British Retail Consortium (BRC); (v) in accordance with food safety system standards (FSSC 22000 – Food Safety System Certification 22000).
For countries or territories that do not issue the certificates specified in Points a, b, and c, Clause 1 of this Article, a certificate or written confirmation must be issued that includes the content of the production facility. Export meets the legal regulations in that country or territory.
According to the Ministry of Health, subjects requiring food safety inspection have changed as follows:
According to regulations before November 9, 2023, food safety inspection when there is duplication or overlap will be carried out according to Joint Circular 13/2014/TTLT-BYT-BNNPTNT-BCT.
However, Joint Circular 13/2014/TTLT-BYT-BNNPTNT-BCT has expired on February 15, 2021, so according to the new regulations in Circular 17/2023/TT-BYT, the principle has been amended the principle for checking food safety when there is duplication or overlap.
If you need advice on food safety regulations, civil law, corporate law, or related amended decrees, please contact Viet An Law Firm for the best support.