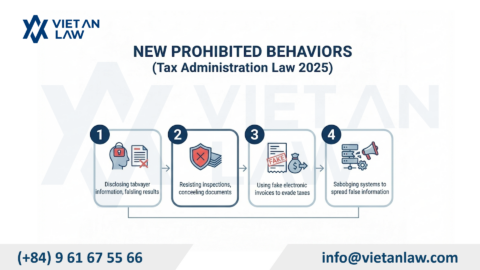
On January 27, 2026, the Government officially issued Resolution No. 66.13/2026/NQ-CP, which regulates Vietnam food regulations 2026, including the food product declaration Vietnam. This document marks a major change in administrative procedures, replacing the “self-declaration” mechanism with “declaration of applicable standards” and setting a mandatory roadmap for businesses to resubmit their applications. To avoid legal risks, understanding Resolution 66.13/2026/NQ-CP: mandatory roadmap for food product re-declaration in Vietnam. In this article, Viet An Law Firm will analyze the new regulations in detail and guide businesses in Vietnam food compliance and food safety accurately and promptly.
Table of contents
This resolution takes effect on the date of issuance, which is January 27, 2026. The regulation is in temporary effect before the 2010 Food Safety Law is officially replaced (the effective date is February 28, 2027) regarding the declaration and registration of food products, aiming to address difficulties during the period awaiting amendment of the law.
The core of this Resolution is to establish a new order for technical standards. Accordingly, food products previously marketed based on old regulations will have to undergo a review and re-declare their products if there are changes in safety indicators. This is a strategic step to synchronize Vietnam’s food safety management system with international standards (Codex, ISO) in the period 2026-2030.
Resolution 66.13/2026/NQ-CP has changed the terminology and management mechanism for common food groups, specifically:
Therefore, businesses no longer have the option of “submitting and selling immediately,” but must wait at least two weeks (15 days) for their applications to be approved.
The process of preparing product registration documents has become more stringent with regulations on testing. The test results certificate must meet two prerequisites:
In addition, the list of mandatory testing parameters has been expanded to include stricter control of residues of new-generation additives and genetically modified organisms (GMOs) for certain sensitive food groups.
Resolution 66.13 takes effect from January 27, 2026. However, to avoid disrupting production and business activities, the Government has established a transitional roadmap (product re-registration roadmap) that business owners need to be aware of at each stage.
Article 15 of the Resolution 66.13/2026/NQ-CP clearly stipulates the transition roadmap for products already in circulation before the Resolution takes effect (January 27, 2026). Businesses need to review their product catalogs to compare them with the two compliance deadlines in Resolution 66.13/2026/NQ-CP as follows:
In terms of legal consequences, after the aforementioned transition period (12 or 24 months) expires, the old documents (Self-declarations or registration certificates) will no longer be valid for production or imported food.
However, to minimize economic losses, the Resolution allows products manufactured during the transitional period to continue circulating until their expiration date.
During this period, businesses need to proactively review their entire product portfolio. They should compare the current basic standards with the new regulations in the Resolution to classify their products:
For businesses with food products requiring re-registration, this is the “golden time” to complete the online application process after declaration of applicable standards and preparing the necessary conditions according to the new regulations. Viet An Law recommends that clients complete the procedures during this period to avoid system congestion at the last minute.
All products manufactured after this date are required to have a declaration of applicable standards as stipulated in Resolution 66.13. Products manufactured during this transitional period will be allowed to circulate until their expiration date. Authorities will conduct specialized inspections and post-inspection campaigns to address violations.
Below is a table comparing the core differences between the new mechanism in your document and the current regulations:
| Criteria | Decree 15/2018/ND-CP (Currently in effect) | Resolution 66.13/2026/NQ-CP (New) |
| Procedure name | Self-declaration of products. | The applicable standards are announced. |
| Business rights | You are entitled to manufacture and market the product immediately after self-declaring (submitting the application/publishing it). | You must wait 15 days after submitting your application. You are only allowed to operate once your application has been published by the regulatory authority (unless you receive a response). |
| Receiving agency | The provincial People’s Committee designates (usually the Department of Health or the Food Safety Management Board) or publishes the information through mass media. | Submit to the state management agency designated by the Provincial People’s Committee. Submission is mandatory so that this agency can publish the information on its website. |
| Testing | Designated or accredited testing laboratory (ISO 17025). | The testing laboratory must be accredited according to ISO/IEC 17025. The test certificate must be valid for 12 months. |
| Dossier’s language | Vietnamese (foreign language documents must be translated and notarized). | • For English documents: You are responsible for translating them into Vietnamese yourself and ensuring the accuracy of the translation.
• For documents in other languages (Chinese, Korean, Japanese, etc.): Notarized translation is mandatory. • Exception for product labels: If the original label is in multiple languages but not English, the business only needs to translate the language of the country of origin or the exporting country. |
To ensure a smooth application process and avoid rejection during the 15-day waiting period, businesses need to prepare a complete set of documents as per Article 6 of the Resolution, including:
Failure to comply with the product re-publication roadmap as stipulated in Resolution 66.13/2026/NQ-CP not only puts businesses at risk of business disruption but also subject them to severe penalties. The 2026 management mechanism will focus strongly on post-inspection and administrative penalties with high fines to deter violations.
Resolution 66.13/2026/NQ-CP: mandatory roadmap for food product re-declaration in Vietnam is a landmark legal document requiring proactive and strict compliance from the business community. With an effective date of January 27, 2026, businesses have a limited time to prepare and transition. Accurately understanding the regulations regarding documentation, testing procedures, and the online application process is key to businesses maintaining a stable supply chain and achieving sustainable development.
Viet An Law Firm, with its extensive experience in food product registration and a team of experts deeply knowledgeable about the latest regulations in 2026, is committed to accompanying businesses in strictly complying with the new regulations, ensuring that your food products circulate legally in the market.