(update Circular 36/2025/TT-BYT dated 11/9/20225)
Good Laboratory Practice (GLP) is a set of principles and standards for a quality management system related to the organization and conditions for conducting non-clinical research in pharmaceutical activities concerning human health and environmental safety, which is planned, implemented, monitored, documented, stored, and reported. The principles of good laboratory practice are stipulated in Circular 04/2018/TT-BYT, amended and supplemented by Circular 08/2020/TT-BYT. Recently, the Ministry of Health granted Circular 36/2025/TT-BYT, amending some articles on the decentralization of Circular 04/2018/TT-BYT, effective from September 11, 2025. In the following article, Viet An Law will update some notable contents regarding regulations on good laboratory practice (GLP) in Vietnam.
Table of contents
According to Article 3, Circular 04/2018/TT-BYT, the principles of good laboratory practice are as follows:
Accordingly, regulated entities include:
According to Article 5, Circular 04/2018/TT-BYT, as amended and supplemented by Clause 1, Article 1 of Circular 08/2020/TT-BYT, documents used as basis for inspection of compliance with GLP by a test facility are those included in its application for certificate of eligibility for pharmacy business (the test facility is not required to submit these documents because they have been submitted when it applies for the certificate of eligibility for pharmacy business) prescribed in Article 38, Law on Pharmacy 2016 and Article 20, Decree 163/2025/NĐ-CP. Specifically, it includes:
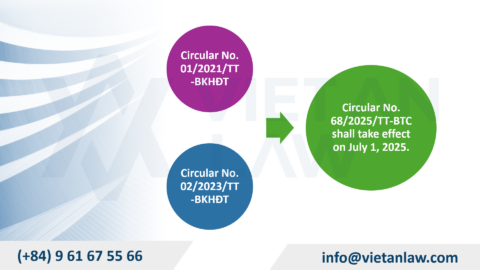
Note: Previously, Article 38, Law on Pharmacy 2016 was guided by Article 32, Decree 54/2017/ND-CP. However, from July 1, 2025, Decree 54/2017/ND-CP has been replaced by Decree 163/2025/ND-CP.
Moreover, documents used as basis for inspection of compliance of a non-commercial test facility with GLP principles include:
In cases where a testing facility requests a GLP certificate along with a certificate of eligibility for pharmacy business, the testing facility must clearly state this in the application for certificate of eligibility for pharmacy business.
According to Clause 1, Article 6, Circular 04/2018/TT-BYT, as amended and supplemented by Circular 08/2020/TT-BYT, the Drug Administration of Vietnam – the Ministry of Health has the authority to inspect the compliance with GLP principles.
In case the facility that only provides bioequivalence study services applies for issuance of the certificate of eligibility for pharmacy business shall submit an application according to regulations of the Minister of Health on good clinical practice.
According to Article 6 of Circular 04/2018/TT-BYT, as amended and supplemented by Circular 08/2020/TT-BYT, procedures for inspection of compliance with GLP principles are as follows:
The test facility shall submit 01 application and pay assessment fees to the Drug Administration of Vietnam – the Ministry of Health.
Procedures for inspection and classification of compliance with GLP principles are as follows:
The degree of compliance of a test facility with GLP principles shall be assessed and includes:
During the interval between periodic inspections, the test facility shall send a notification of change made using the Form No. 05 in the Appendix V attached to Circular 04/2018/TT-BYT in one of the following cases:
Accordingly, previously, Clause 4, Article 11 of Circular 04/2018/TT-BYT stipulated that test facilities undergoing changes falling under one of the above cases shall submit a notification of change enclosed with technical documents corresponding to the change to the Drug Administration. The Drug Administration of Vietnam shall assess the notification of change sent by the test facility.
However, this regulation was amended by Clause 1, Article 1 of Circular 36/2025/TT-BYT, effective from September 11, 2025, as follows: “The test facility that makes one of the changes specified in points d, dd and e clause 1 of this Article shall submit a change report enclosed with relevant technical documents to the Department of Health of the province where the laboratory is located. The Department of Health shall assess the change report sent by the test facility.”
Therefore, according to this new regulation, the authority to assess the notification of change of the test facility will be transferred from the the Drug Administration of Vietnam – the Ministry of Health to the Department of Health in the locality where the laboratory is located. At the same time, the processing time for applications is also stipulated as follows:
Previously, according to Circular 04/2018/TT-BYT, the deadline for issuing a notification agreeing to the changes, if the changes met the requirements, was 10 days, and there was no regulation regarding the publication of the notification agreeing to the changes on the website.
Clause 2, Article 1 of Circular 36/2025/TT-BYT amends the regulation in Clause 2, Article 18, Circular 04/2018/TT-BYT regarding the responsibilities of the Department of Health concerning good laboratory practice as follows:
This new regulation has added the responsibility to assess change reports from testing facilities and publish them on the unit’s website, and to send a notification to the Ministry of Health (the Drug Administration of Vietnam) confirming agreement with the changes, in order to align with the Ministry of Health’s new authority to assess the notification of change of the test facility.
The above is an update on the regulations on good laboratory practice (GLP) in Vietnam. If you have any related questions or require legal advice regarding the assessment of compliance with good laboratory practice, please contact Viet An Law for the best advice and support!