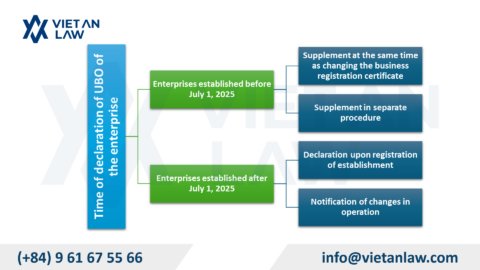
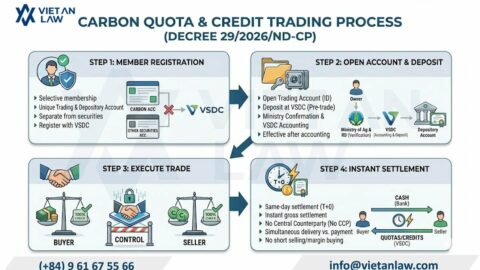
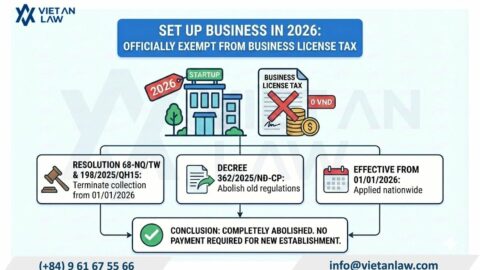
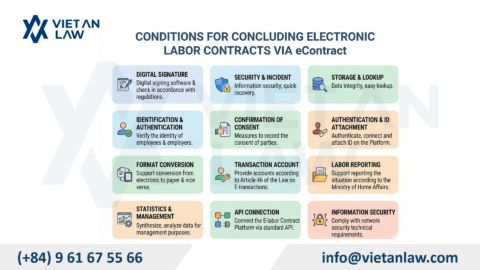
According to Annex 1 of the cosmetic group illustration of the Agreement on the Asean harmonized cosmetic regulatory scheme, the perfume have to carry out cosmetic procedures prior to circulation. However, with composition, physical form and personal use, cosmetic procedures for perfume and cosmetic products also differ.
Generally speaking, the publication of cosmetic products is not only understood as the process of preparation of dossiers, the application for certificates of the circulation of cosmetics, but the filing of dossiers for inspection by the inspectorates. Examine and statistics the authenticity of products and licenses equivalent to individuals and organizations. Specific steps to take steps to publish cosmetics is conducted as follows:
Step 1: The manner to establish the cosmetic product proclamation report and the proclamation data
The cosmetic product report is set up in accordance with the form in appendix No 01-MP. The manner to write contents in the cosmetic product report shall followed the instruction in the appendix No 02-MP. Details are:
Products have the same owners in one of the following situations is permitted to proclaim in one proclamation report:
Product features:
The manner to write component of formula which are composed in the cosmetic product:
Ingredient’s name must be written in the International law name Nomenclature of Cosmetic Ingredients – INCI is regulated in the latest publications.
The language in the proclamation report may be in Vietnamese or English.
The organization or the individual, whose name is on the cosmetic proclamation, must completely be responsible for the propriety of content of the cosmetics product proclamation report (signed and sealed version) compare with the proclamation data (soft version) declared or submitted to the Management Office.
Step 2: The manner to set up registration file and the order procedure of cosmetic product announcement
In case of omestic cosmetic:
Organizations or individuals are responsible of putting products on the market who are the manufacturer, Cosmetic product owner
Organizations or individuals are responsible of putting products on the market who are not the manufacturer, cosmetic product owner:
In case of import cosmetic product:
Certificate of free sale (CFS) is only applied for import cosmetic product proclamation which satisfies the following requirements:
CFS which is issued by the current territory must have been original or legally notarized and still in the day of validity. In case CFS is not provided of the expiry day, it must be a certificate which has just been issued within 24 months; and
CFS must be consul legalized according to provisions of the law, except consul legalization immunity case according to the international treaties in which Vietnam is a member.
Step 3: Save the Product Information File (PIF)
Every cosmetic product must get a Product Information File (PIF) when delivered to the market in accordance with ASEAN’s instruction which is saved at the address of organizations or individuals who are responsible for putting the product on the market.
The Product Information File involves of the following 04 parts:
Part 1: Administrative documents and a summary of product;
Part 2: Material quality;
Part 3: Product quality; and
Part 4: Safety and efficiency.
The details of the product information file are regulated in Appendix 07-MP.
Part 1 of the Product Information File must immediately be presented to the agency of Consideration, Investigation when required; others, if inefficiently, must be presented within 15-60 days since the consideration day in regard to the Functional agency’s request.
Procedure
Within 20-30 working days as from the date of receiving the complete dossiers of announcement of cosmetic products, department of Medicine management – the Health Ministry shall have to promulgate the number of publicized products. After receiving the number of products announced, the products are free to circulate on the territory of Vietnam.
Results of settling of dossiers of announcement of cosmetic products: The competent state agencies shall issue decisions or refuse to issue the serial number of reports on cosmetic products.
The receipt number of cosmetic product proclamation report shall be valid for 05 years since issuing day. After 05-year-expiry, if organizations or individuals want to continue selling product in the market, they must make a proclamation again before the expiry of the receipt number of cosmetic product proclamation report and pay a regulated cost fee.